GSPRs are the General Safety and Performance Requirements for CE Marking of medical devices and IVDs in Annex I of the EU MDR and IVDR.

General Safety and Performance Requirements (GSPRs) are the requirements for safety and performance specified in Annex I of the EU MDR and EU IVDR. GSPRs are divided into Chapter I (i.e., – Sections 1-9 of the MDR and Sections 1-8 of the IVDR are the General requirements), Chapter II (i.e., – Sections 10-22 of the MDR and Sections 9-19 of the IVD are the Requirements regarding design and manufacture), and Chapter III (i.e., Section 23 of the MDR and Section 20 of the IVDR are the requirements regarding the information supplied with the device or IVD). All devices must meet the requirement of Chapter I and Chapter III, but the applicability of Chapter II depends upon the technological characteristics of the device or IVD.
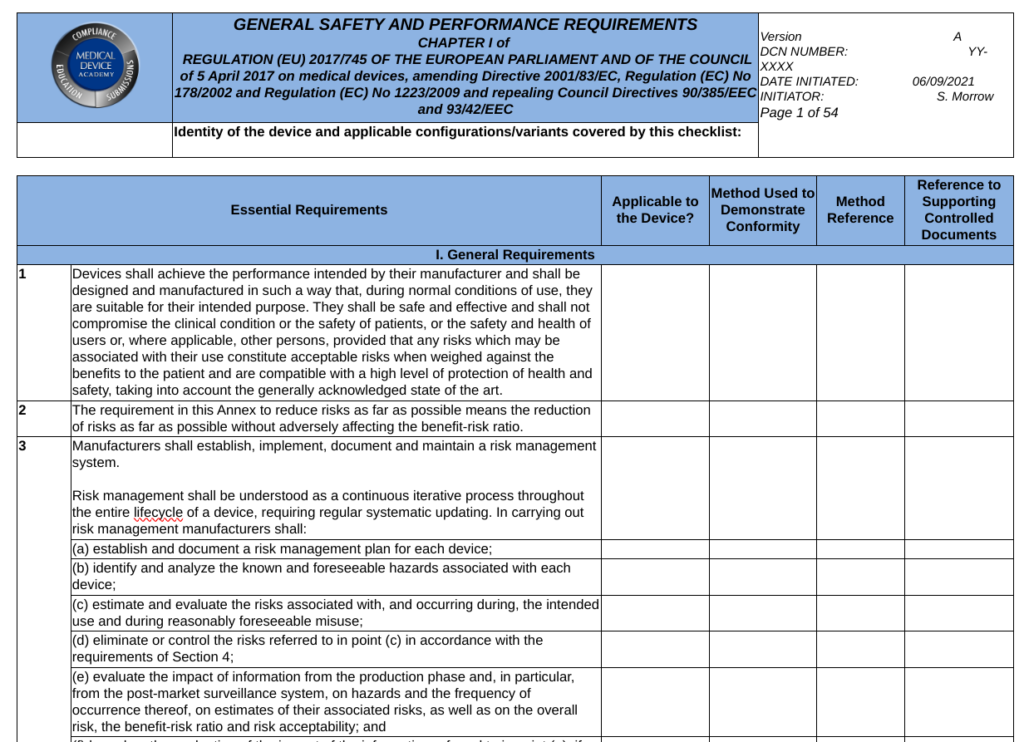
When a Notified Body reviews your technical documentation, they expect you to provide either a complete technical file or technical file index that is organized in accordance with Annex II of the MDR or IVDR. Section 4 of Annex II is labeled “General Safety and Performance Requirements.” This section is where your GSPR checklist should be located in the technical file or technical file index. Generally a GSPR checklist is considered the best way to document these requirements. The checklist should should provided traceability to each specific requirement and the following elements:
The general requirements for safety and performance (i.e., Chapter I in Section 1-9 of the MDR and Section 1-8 of the IVDR) are primarily focused on risk management requirements. These first few sections state that the manufacturer must ensure that the device or IVD is safe, effective, and does not compromise the clinical condition or safety of patients or users. The manufacturer must take into account the generally acknowledged state of the art. Risks must be reduced as far as possible without adversely affecting the benefit-risk ratio. The manufacturer must implement a risk management system. Risks associated with use errors shall be eliminated or reduced as far as possible. The characteristics of performance shall not be adversely affected the conditions of use, transport and storage during the lifetime of the device or IVD. Finally, all residual risks shall be minimized and be acceptable when weighted against the benefits to the patients and/or user arising from the intended use during normal conditions of use.
This Chapter of the GSPRs is organized into the following subsections of the MDR:
This Chapter of the GSPRs is organized into the following subsections of the IVDR:
Chapter III is divided into four subparts. Section 23.1 of the MDR and section 20.1 of the IVDR are the general requirements for information provided by the manufacturer (i.e., labeling). The recommended harmonized standard is EN ISO 20417:2021. Section 23.2 of the MDR and section 20.2 of the IVDR include the labeling requirements. Section 23.3 of the MDR and section 20.3 of the IVDR include requirements for information on the packaging which maintains the sterile condition of a device or IVD (i.e., label on the sterile barrier packaging). Finally, Section 23.4 of the MDR and section 20.4 of the IVDR include requirements for the Instructions for Use (i.e., IFU, Directions for Use, or User Manual).
Completing the GSPR Checklist would be easy if there were only 20-23 requirements, but most of the requirements have multiple requirements. For example, GSPR 14 of the MDR has 7 subparts, 18 of the MDR has 8 subparts, and labeling requirements are six pages long. Each subpart must be addressed when you complete the columns of the checklist. If any of the parts or subparts do not apply to your device, you need to provide a justification. When you write your justification for the non-applicability of a GSPR, you need to be careful to provide a justification for each subpart of the requirement–even if the subpart is not separately identified by a letter or number.
If you need a template for creating your own GSPR checklist, you can download our template by filling in the form below:
Health Canada also identifies Essential Principles for Safety and Effectiveness in Sections 10-20 of the Canadian Medical Device Regulations (i.e., SOR 98/282) that is similar to the European GSPRs, and Australia has a similar Essential Principles Checklist document with only a few minor differences. The Global Harmonized Task Force (GHTF) created an earlier version in 2005, but the International Medical Device Regulators Forum (IMDRF) released a newer version in 2024. Health Canada will typically accept your GSPR checklist developed for CE Marking, but a gap analysis should be performed against the Australian Regulations.
Hi! I would like to download the free template for the new essential requirements, but it seems that the link is broken( http://bit.ly/NewERCTemplate) . Can you please help guide me to where I can download this document? Thanks so much!
Rob PackardDear Laina,
Thank you for your request. I could send you the template we created a few years ago, but it is being updated for the final release of the MDR that was published in May 2017. The gap analysis we did earlier was for an early draft. I just completed a 5-day training of a Notified Body team of auditors last week, and we are updating much of our documentation for compliance with the new MDR. Please watch for new blogs which will be coming soon on this topic.
Hi Rob, I have just checked out your website and the MDR Essentials Principles checklist is not on there to download. Would you be able to send me a copy?
I have just started as a consultant in the Medical Device field after 25 years working in the industry. Not sure I picked a good time to go solo!
I’m working on MDR and MDSAP training and implementation for a couple of companies.
Many thanks Jane Oglesby
Hi Jane,
Autoresponders for downloading the checklist don’t always work, but I just sent you an email with what you are looking for. Don’t worry too much about your timing to go solo. It’s not about when it’s good in general, but when it’s good for you personally. Please let me know if I can help you in any way.
Rob
Hi rob, the link for downloading template is broken.
can you give me a link that works so I can download the template.
we are interested in selling masks and I wonder why the checklist will say what we must keep in mind to sell these masks. Many thanks, henk
Thank you for the comment. I haven’t updated the entire article, but the link to download the ERC checklist for the MDD and the new MDR has been repaired.
Rob
This site uses Akismet to reduce spam. Learn how your comment data is processed.